Nubeqa contains the active substance darolutamide. The safety and efficacy of NUBEQA have not been established in females.
Https 1au3b422k9zdqzddw3my51gg Wpengine Netdna Ssl Com Wp Content Uploads Sites 7 2019 08 Nubeqa S Pdf
They inhibit the binding of aminoacyl-tRNA to the mRNA translation complex.

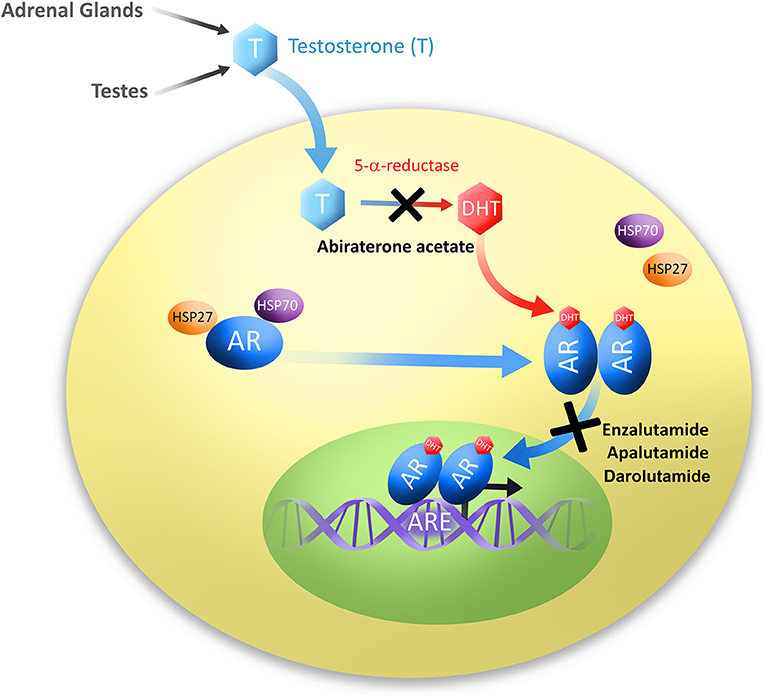
Nubeqa mechanism of action. Nubeqa is contraindicated in women who are or may become pregnant. Darolutamide competitively inhibits androgen binding AR nuclear translocation and AR mediated transcription. Nubeqa interferes with the ability of male hormones to bind to their receptors within a cell and also reduces the ability of the receptors to enter the nucleus and stimulate cell growth.
Mechanism of action. NUBEQA is not indicated in women. Sofosbuvir inhibits the hepatitis C NS5B protein.
NUBEQA is indicated for the treatment of adult men with non-metastatic castration resistant prostate cancer nmCRPC who are at high risk of developing metastatic disease see section 51. 63 Drug Labels for Ingredients. It works by blocking the effects of.
The mechanism of action of Nubeqa is to block the effects of testosterone. They inhibit the initiation of translation in variety of ways by binding to the 30S ribosomal subunit which is made up of 16S rRNA and 21 proteins. Animal embryo fetal toxicology studies have not been performed.
Tetracycline antibiotics are protein synthesis inhibitors. Sofosbuvir is a prodrug of the Protide type whereby the active phosphorylated nucleotide is granted cell permeability and oral bioavailability. Sofosbuvir appears to have a high barrier to the development of resistance.
Nubeqa is indicated for the treatment of adult men with non metastatic castration resistant prostate cancer nmCRPC who are at risk of developing metastatic disease European Medicines Agency EMA 62 FDA Orange Book. How is Nubeqa given administered. There are no data available with the use of Nubeqa during pregnancy in humans.
Darolutamide competitively inhibits androgen binding AR nuclear translocation and AR-mediated transcription. Nubeqa belongs to a group of medicines called androgen receptor inhibitors. There are no human data on the use of NUBEQA in pregnant women.
42 Posology and method of administration. By blocking these hormones darolutamide stops prostate cancer cells from growing and dividing. However based on the mechanism of action NUBEQA can cause embryofetal harm or loss of pregnancy.
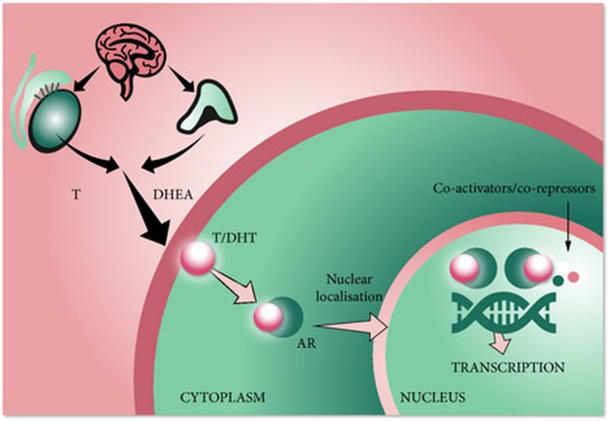
Based on its mechanism of action Nubeqa may cause fetal harm when administered during pregnancy. Prostate cancer depends on testosterone to grow and spread. Nubeqa is a medicine used to treat men with prostate cancer.
It is used when the cancer is castration-resistant worsens despite treatment to lower testosterone levels including surgical removal of the testes and is at high risk of metastasis spreading to other parts of the body. NUBEQA- darolutamide tablet film coated. This medication belongs to a class of drugs known as anti-androgens anti- testosterone.
It works by blocking the activity of male sex hormones called androgens such as testosterone leading to inhibition of growth and division of prostate cancer cells. Therefore NUBEQA is not to be used in women who are or may becomepregnant. Darolutamide is used to treat a certain type of prostate cancer.
Darolutamide is an androgen receptor AR inhibitor with a flexible polar-substituted pyrazole structure that binds with high affinity directly to the receptor ligand binding domain. What is the mechanism of action. 121 Mechanism of Action Darolutamide is an androgen receptor AR inhibitor.
Based on its mechanism of action NUBEQA can cause fetal harm and loss of pregnancy when administered to a pregnant female.
 Fda Approves Bayer S Nubeqa Darolutamide A New Treatment For Men With Non Metastatic Castration Resistant Prostate Cancer
Fda Approves Bayer S Nubeqa Darolutamide A New Treatment For Men With Non Metastatic Castration Resistant Prostate Cancer
 Nubeqa Darolutamide Tablets Uses Dosage Side Effects Interactions Warning
Nubeqa Darolutamide Tablets Uses Dosage Side Effects Interactions Warning
 Prostate Cancer And Non Metastatic Castration Resistant Prostate Cancer Read Slide Landscape Overview Ppt Download
Prostate Cancer And Non Metastatic Castration Resistant Prostate Cancer Read Slide Landscape Overview Ppt Download
 Frequently Asked Questions About Nubeqa Darolutamide Cancerconnect
Frequently Asked Questions About Nubeqa Darolutamide Cancerconnect
 Fda Approves Bayer S Nubeqa Darolutamide A New Treatment For Men With Non Metastatic Castration Resistant Prostate Cancer
Fda Approves Bayer S Nubeqa Darolutamide A New Treatment For Men With Non Metastatic Castration Resistant Prostate Cancer
 Frontiers Second Generation Antiandrogens From Discovery To Standard Of Care In Castration Resistant Prostate Cancer Oncology
Frontiers Second Generation Antiandrogens From Discovery To Standard Of Care In Castration Resistant Prostate Cancer Oncology
Https Www Amcp Org Sites Default Files 2019 12 Mac Nub Us 0020 2 20nubeqa 20presentation 20for 20amcp 20webinar Pdf
 Darolutamide Delays The Spread Of Some Prostate Cancers National Cancer Institute
Darolutamide Delays The Spread Of Some Prostate Cancers National Cancer Institute
 Prostate Cancer And Non Metastatic Castration Resistant Prostate Cancer Read Slide Landscape Overview Ppt Download
Prostate Cancer And Non Metastatic Castration Resistant Prostate Cancer Read Slide Landscape Overview Ppt Download
Https S27 Q4cdn Com 906368049 Files News 2019 Zacks Scr Research 08262019 Epix Bautz Pdf
Https Www Ema Europa Eu En Documents Assessment Report Nubeqa Epar Public Assessment Report En Pdf
Https Www Amcp Org Sites Default Files 2019 12 Mac Nub Us 0020 2 20nubeqa 20presentation 20for 20amcp 20webinar Pdf
 Nubeqa Darolutamide Clinical Information
Nubeqa Darolutamide Clinical Information

Comments
Post a Comment