In August 2017 the US FDA approved CPX-351 vyxeos a liposomal formulation of cytarabine and daunorubicin at a fixed 51 molar ratio for the treatment of adults with newly diagnosed AML with myelodysplasia-related changes AML-MRC and therapy-related AML t-AML. Histone deacetylase inhibitors may synergize with hypomethylating agents.
 Interpretation Of Clinical Endpoints In Trials Of Acute Myeloid Leukemia Sciencedirect
Interpretation Of Clinical Endpoints In Trials Of Acute Myeloid Leukemia Sciencedirect
The primary end point was overall survival.
Vyxeos clinical trial. In this open-label randomized phase III trial 309 patients age 60 to 75 years with newly diagnosed high-risksAML received one to two induction cycles of CPX-351 or 73 followed by consolidation therapy with a similar regimen. We report final results from a randomized open-label study of first-line CPX-351 in patients with high. Older patients with secondary AML have poor outcomes following first-line cytarabine and anthracycline-based treatment.
The complete response rate was 38 in the Vyxeos-treated group and 26 in the control group. CPX-351 is a liposomal formulation of cytarabine and daunorubicin encapsulated at a 51 molar ratio with enhanced efficacy among poor risk AML patients. The safety of VYXEOS was determined in a randomized trial for adults with newly-diagnosed tAML or AML-MRC see Clinical Studies which included 153 patients treated with VYXEOS and 151 patients treated with a standard combination of cytarabine and daunorubicin 73.
Age 18 years. CPX-351 Vyxeos for Transplant Eligible Higher Risk Patients With Myelodysplastic Syndrome - Full Text View - ClinicalTrialsgov CPX-351 Vyxeos for Transplant Eligible Higher Risk Patients With Myelodysplastic Syndrome The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. This is a single-institution Phase I pilot study designed to test the safety and tolerability of combining venetoclax with Vyxeos CPX-351 cytarabine and daunorubicin liposome for the treatment of relapsedrefractory acute leukemia in young patients.
The decision to prescribe VYXEOS must have been made prior to enrollment in this study and based upon approved US indications and dosing. Daunorubicin and cytarabine liposome for injection a dual-drug liposomal encapsulation of cytarabine C and daunorubicin D is approved by the FDA and EMA for the treatment of adults with newly diagnosed therapy-related AML or. This is a single-institution Phase I pilot study designed to test the safety and tolerability of combining venetoclax with Vyxeos CPX-351 cytarabine and daunorubicin liposome for the treatment of relapsedrefractory acute leukemia in young patients.
This study will use what is called a liposome injection. Prior to initiating each cycle of VYXEOS calculate the. Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted.
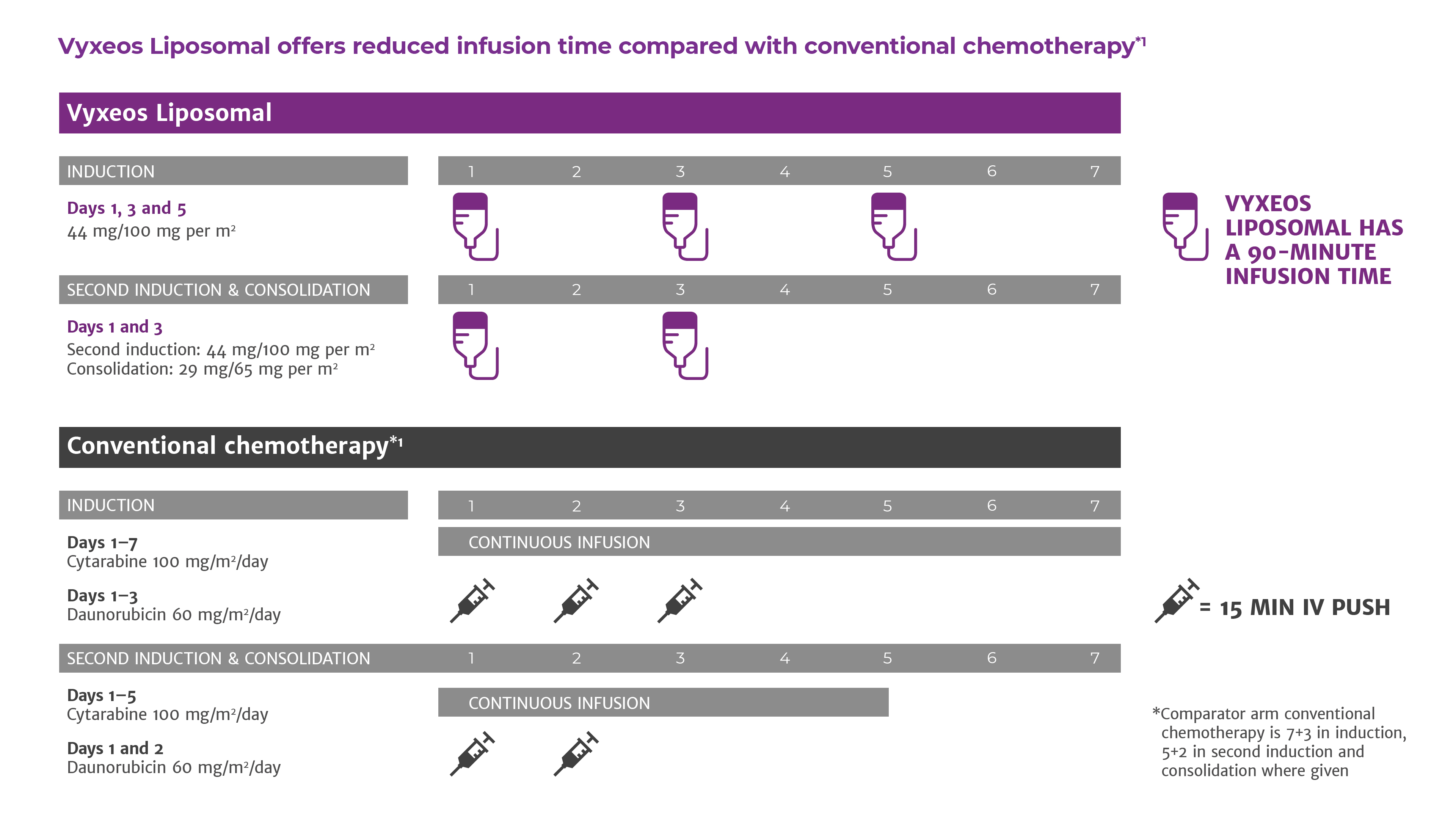
Description This phase II trial studies the side effects and how well Vyxeos works in treating patients with intermediate and high-risk acute myeloid leukemia who have failed an initial cycle of standard cytarabine and daunorubicin chemotherapy. A phase 1 clinical trial of vorinostat in combination with decitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome Patients with acute myeloid leukaemia AML or myelodysplastic syndrome MDS may respond to treatment with epigenetic-modifying agents. This study involves Vyxeos CPX-351 a formulation of a fixed combination of the two anti-tumor drugs cytarabine and daunorubicin that will be given as an infusion over 90 minutes.
44mgm2 daunorubicin and cytarabine 100 mg2 on Days 1 3 and 5. Vyxeos is a combination of both chemotherapy drugs cytarabine and daunorubicin contained in a liposome. Vyxeos is a combination of both chemotherapy drugs cytarabine and daunorubicin contained in a liposome.
A full VYXEOS course consists of 1-2 cycles of Induction and up to 2 cycles of Consolidation at the dose and schedule listed in Table 1. Results of the trial presented at the 2016 American Society of Clinical Oncology annual meeting showed that median overall survival was 96 months in patients who received Vyxeos compared with 59 months in the control group. This phase II trial studies the side effects and how well Vyxeos works in treating patients with intermediate and high-risk acute myeloid leukemia who have failed an initial cycle of standard cytarabine and daunorubicin chemotherapy.
Daunorubicin cytarabine. Ability to understand and voluntarily give informed consent and understand the requirements of the registry. Study Design The FDA approval of VYXEOS CPX-351 was based on data from a large pivotal Phase 3 study 1 The Phase 3 study was a multicenter open-label active-controlled randomized trial of VYXEOS vs 73a in 309 patients aged 60-75 with newly-diagnosed t-AML or AML-MRC2.
Social Media Monitoring On Vyxeos Research And Study On Social Media Channels
 Vyxeos Improves Survival For Elderly In Aml Cancerconnect
Vyxeos Improves Survival For Elderly In Aml Cancerconnect
 Cpx 351 A Nanoscale Liposomal Co Formulation Of Daunorubicin And Cyta Ijn
Cpx 351 A Nanoscale Liposomal Co Formulation Of Daunorubicin And Cyta Ijn
 Vyxeos Chemotherapy Liposome Injection For Acute Myeloid Leukemia Ppt Download
Vyxeos Chemotherapy Liposome Injection For Acute Myeloid Leukemia Ppt Download
 Vyxeos Chemotherapy Liposome Injection For Acute Myeloid Leukemia Ppt Download
Vyxeos Chemotherapy Liposome Injection For Acute Myeloid Leukemia Ppt Download
 Vyxeos Scores A 10 Over 7 3 Obr
Vyxeos Scores A 10 Over 7 3 Obr
 Vyxeos Chemotherapy Liposome Injection For Acute Myeloid Leukemia Ppt Download
Vyxeos Chemotherapy Liposome Injection For Acute Myeloid Leukemia Ppt Download
 Celator Pharmaceuticals Progressing The Paradigm Of Combination Chemotherapy Nasdaq Cpxx Seeking Alpha
Celator Pharmaceuticals Progressing The Paradigm Of Combination Chemotherapy Nasdaq Cpxx Seeking Alpha
Vyxeos Phase 3 Clinical Trial Study Design Vyxeos Daunorubicin And Cytarabine Hcp
 Vyxeos Chemotherapy Liposome Injection For Acute Myeloid Leukemia Ppt Download
Vyxeos Chemotherapy Liposome Injection For Acute Myeloid Leukemia Ppt Download
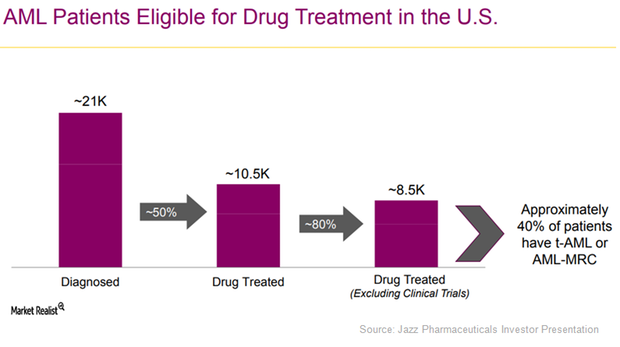 What Jazz Pharmaceuticals Expects For Vyxeos
What Jazz Pharmaceuticals Expects For Vyxeos
Https Investor Jazzpharma Com Static Files 5405fd93 36bb 49c5 B152 B8d7a07d9bfe
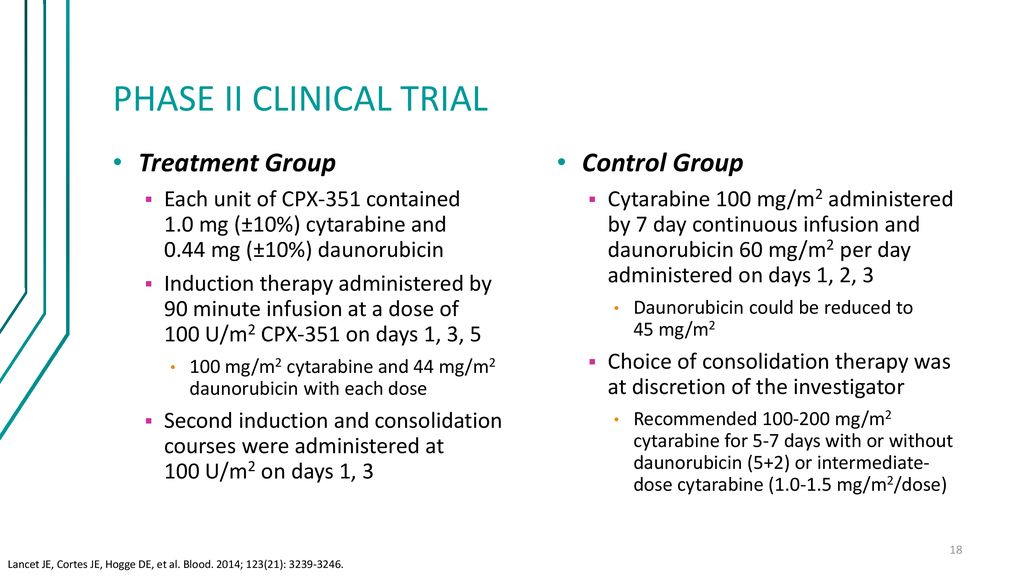 Vyxeos Chemotherapy Liposome Injection For Acute Myeloid Leukemia Ppt Download
Vyxeos Chemotherapy Liposome Injection For Acute Myeloid Leukemia Ppt Download
Comments
Post a Comment