According to the FDAs clinical trials on Ubrelvy the most common side effects to watch for include nausea or upset stomach drowsiness and fatigue. Participation in another clinical trial involving an investigational drug within the last 30 days prior to baseline.
 Drug Trials Snapshots Ubrelvy Fda
Drug Trials Snapshots Ubrelvy Fda
UBRELVY also met co-primary endpoints of freedom from pain and freedom from the most bothersome symptom nausea hypersensitivity to light or hypersensitivity to sound a recent more stringent standard of efficacy the FDA set in 2018.

Ubrelvy clinical trials. Hutchinson S Dodick DW Treppendahl C Bennett NL Yu SY Guo H Trugman JM. Actual Primary Completion Date. A Phase 1b Two-Part Open-Label Fixed-Sequence Safety Tolerability and Drug-Drug Interaction Study Between Single Dose Erenumab or Galcanezumab and Multiple Dose Ubrogepant in Participants With Migraine.
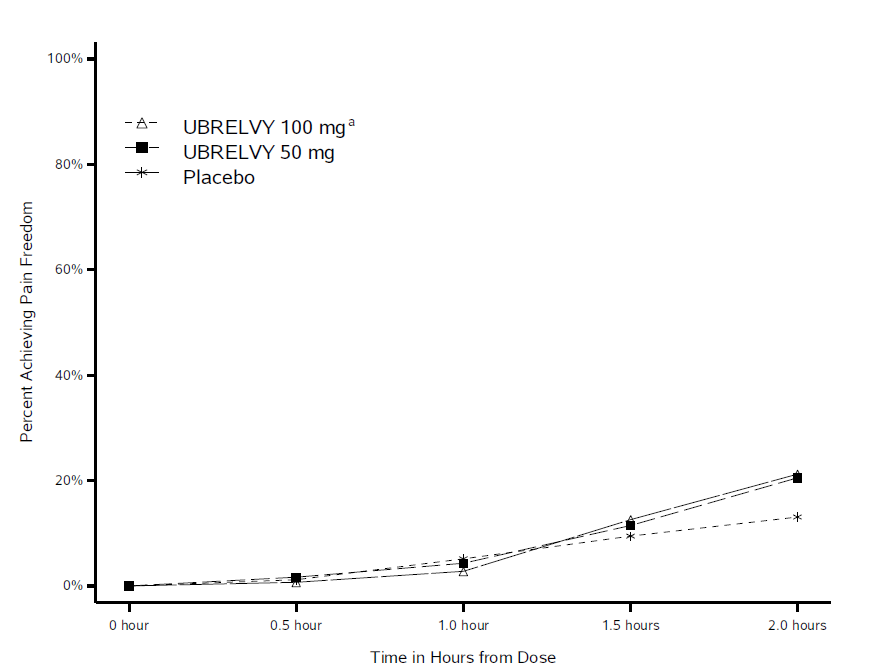
The coprimary efficacy. In clinical trials supporting the FDAs approval UBRELVY provided quick pain relief for the majority of migraine patients. The safety of UBRELVY was evaluated in.
A Double-Blind Placebo Controlled Crossover Trial of BHV-3000 Rimegepant for Treatment Refractory Trigeminal Neuraligia. Botox is prescribed for the prevention of headaches in adults diagnosed with chronic migraine. Resource links provided by the National Library of.
Design setting and participants. Antimigraine agent Calcitonin Gene-Related Peptide CGRP Receptor Antagonist. Phase 3 multicenter randomized double-blind placebo-controlled single-attack clinical trial ACHIEVE II conducted in the United States 99 primary care and research clinics.
UBRELVY provided lasting relief up to 24 hours as well. Pooled Efficacy Safety and Tolerability From the ACHIEVE I and ACHIEVE II Phase 3 Randomized Trials. Who participated in the clinical trials.
Clinical Review Laura Jawidzik MD NDA 211765 UbrogepantUBRELVY A deficiency in the release of CGRP has been implicated in the lack of vasodilatation observed in Raynauds phenomenon and administration of CGRP has been shown to have a. Triple Participant Care Provider Investigator Primary Purpose. August 26 2016-February 26 2018.
Use of any medication that is a strong or moderate inhibitor of CYP3A a strong or moderate inducer of CYP3A or an inhibitor of glycoprotein P-gp or Breast Cancer. A Worldwide Randomized Double Blind Placebo-Controlled Parallel Group Clinical Trial to Evaluate the Safety and Efficacy of Rizatriptan for the Acute Treatment of Migraine in Children and Adolescents. Treatment of migraine with or without aura in adults.
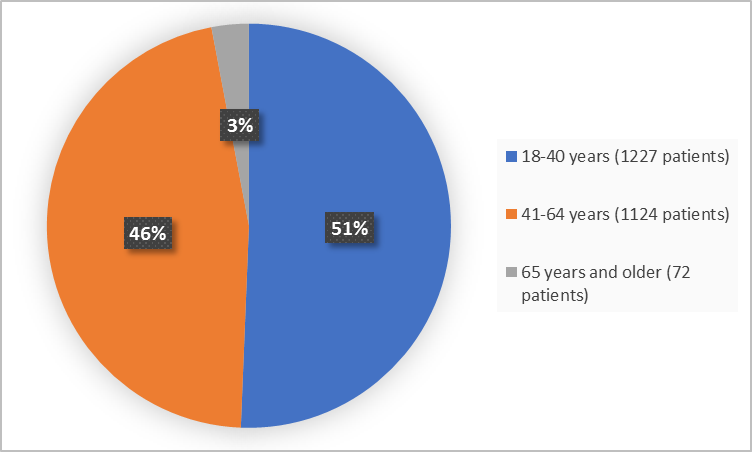
Participants were adults with migraine with or without aura experiencing 2 to 8 migraine attacks per month. Actual Study Completion Date. Clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
However the entrance of atogepant. Manufacturer1 Allergan USA Inc. The FDA approved UBRELVY based on evidence from two clinical trials Trial 1NCT02828020 and Trial 2NCT02867709 of.
Actual Study Start Date. Ubrogepant sold under the trade name Ubrelvy is a medication used for the acute immediate treatment of migrainewith or without aura a sensory phenomenon or visual disturbance in adults1 It is not indicated for the preventive treatment of migraine1. Epub ahead of print.
Actual Study Start Date. Ubrogepant for the Acute Treatment of Migraine. We conducted a randomized trial to evaluate the efficacy safety and side-effect profile of ubrogepant.
None Open Label Primary Purpose. Inability for woman of child-bearing potential to use an effective form of contraception if sexually active. The potential approval of atogepant will expand AbbVies portfolio of marketed migraine therapeutics which consists of Ubrelvy and Botox onabotulinumtoxin A.
Interventional Clinical Trial Actual Enrollment. Ubrelvy is used for acute migraine treatment and has not been investigated for migraine prevention. Double Participant Investigator Primary Purpose.
Interventional Clinical Trial Estimated Enrollment. A Phase 3 Multicenter Randomized Double-Blind Placebo Controlled Single Attack Study to Evaluate the Efficacy Safety and Tolerability of Oral Ubrogepant in the Acute. We assigned adults with migraine with or without aura in a 111 ratio to receive an initial dose of placebo ubrogepant at a dose of 50 mg or ubrogepant at a dose of 100 mg for treatment of a single migraine attack with the option to take a second dose.
Effective Migraine Relief Ubrelvy Ubrogepant Hcp
 Allergan Receives U S Fda Approval For Ubrelvy For The Migraine Treatment
Allergan Receives U S Fda Approval For Ubrelvy For The Migraine Treatment
 Drug Trials Snapshots Ubrelvy Fda
Drug Trials Snapshots Ubrelvy Fda
 Allergan Receives U S Fda Approval For Ubrelvy For The Migraine Treatment
Allergan Receives U S Fda Approval For Ubrelvy For The Migraine Treatment
 Ubrogepant Cgrp Education Research Forum
Ubrogepant Cgrp Education Research Forum
 What Is Ubrelvy Ubrogepant Acute Treatment For Migraine Attacks
What Is Ubrelvy Ubrogepant Acute Treatment For Migraine Attacks
 Ubrogepant An Acute Treatment For Migraine Improved Patient Reported Functional Disability And Satisfaction In 2 Single Attack Phase 3 Randomized Trials Achieve I And Ii Dodick 2020 Headache The Journal Of
Ubrogepant An Acute Treatment For Migraine Improved Patient Reported Functional Disability And Satisfaction In 2 Single Attack Phase 3 Randomized Trials Achieve I And Ii Dodick 2020 Headache The Journal Of
 Ubrogepant An Acute Treatment For Migraine Improved Patient Reported Functional Disability And Satisfaction In 2 Single Attack Phase 3 Randomized Trials Achieve I And Ii Dodick 2020 Headache The Journal Of
Ubrogepant An Acute Treatment For Migraine Improved Patient Reported Functional Disability And Satisfaction In 2 Single Attack Phase 3 Randomized Trials Achieve I And Ii Dodick 2020 Headache The Journal Of
 Drug Trials Snapshots Ubrelvy Fda
Drug Trials Snapshots Ubrelvy Fda
Https 1au3b422k9zdqzddw3my51gg Wpengine Netdna Ssl Com Wp Content Uploads Sites 7 2020 02 Ubrelvy Pdf
 Drug Trials Snapshots Ubrelvy Fda
Drug Trials Snapshots Ubrelvy Fda
 Drug Trials Snapshots Ubrelvy Fda
Drug Trials Snapshots Ubrelvy Fda
 Drug Trials Snapshots Ubrelvy Fda
Drug Trials Snapshots Ubrelvy Fda

Comments
Post a Comment